.svg) National Institute of General Medical Sciences |
 |
 |
National Biomedical Resource for |
| ACERT's Service and Collaborative Projects | |
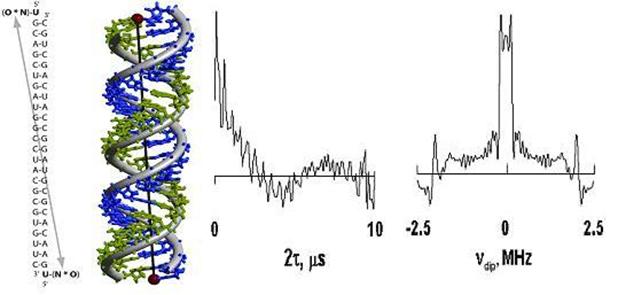
Double quantum coherence (DQC)-ESR has recently been shown to be a powerful technique for distance measurements in proteins. It yields high quality dipolar spectra, and it significantly extends the range of measurable distances in proteins with the use of double nitroxide labeling. However, it is difficult to extend this range much beyond the 50 Å limit, because the acquisition time required to discern the dipolar oscillation, (whose frequency is inversely proportional to the cube of the distance between the spin labels), becomes long compared to the electron-spin phase memory time, Tm. For labeled sites exposed to the frozen aqueous solvent, phase relaxation is largely (although not entirely) determined by nuclear-spin-diffusion of the matrix protons, which modulate their many-body dipolar interactions with the electron spin. By a variant of the DQC-ESR method, we are able to improve on the partial suppression of the effects of nuclear-spin-diffusion on the electron spin phase-memory time, and thereby enable the accurate measurement of considerably larger distances, of about 70 Å. This variant employs the same six-pulse sequence, but in a format which simply refocuses the primary echo, after it is put through a double quantum filter. Thus we refer to this method as Double-Quantum Filtered Refocused (DQFR)-Electron Spin Echoes (ESE). We have demonstrated its use for long distances on a long double-stranded A-type RNA consisting of 26 base-pairs (See Figure), that was spin-labeled at both ends. We estimate a distance of 70±5 Å between the two end labels from molecular modeling. The samples for ESR were in deuterated solvent, which is known to suppress the effects of nuclear-spin diffusion, and corresponded to about 1nmole of doubly labeled RNA. The resulting signal is shown below. It clearly displays the dipolar oscillation over a 10 µs. period which corresponds to a doubling of this interval, as compared to DQC-ESR. A spectrum is obtained, yielding a dipolar splitting, νdip=140 kHz, corresponding to a distance, r = 72±4 Å between the end-labeled nitroxides, in excellent agreement with molecular modeling. Such a result could not have been reliably obtained from the DQC-ESR spectrum. The DQFR-ESE method thus effectively extends DQC-ESR to longer distances by providing a longer interval of signal acquisition. Publication: P.P. Borbat, J.H. Davis, S.E. Butcher, and J.H. Freed, J. Am. Chem. Soc., 126, 7746-7747 (2004); no PMCID |
|
 |
|
|
Peter P. Borbat, Jack H. Freed (ACERT) Jared H. Davis, Samuel E. Butcher, (University of Wisconsin-Madison) |
|
|
|
About ACERT Contact Us |
Research |
Outreach |
ACERT is supported by grant 1R24GM146107 from the National Institute of General Medical Sciences (NIGMS), part of the National Institutes of Health. |
|||||
| ||||||||