.svg) National Institute of General Medical Sciences |
 |
 |
National Biomedical Resource for |
| ACERT's Service and Collaborative Projects | ||
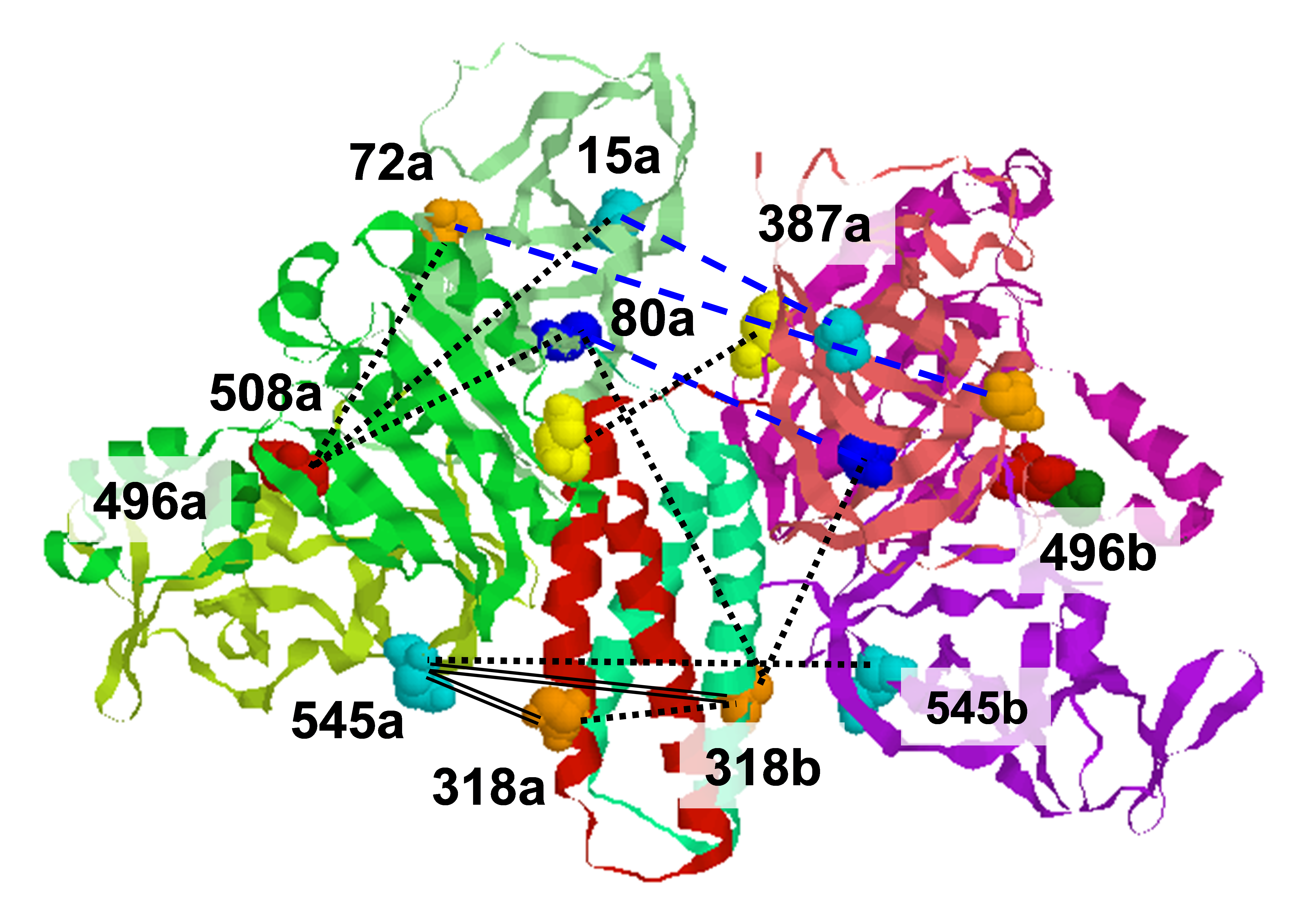
Proteins are dynamic entities that populate conformational ensembles, and most functions of proteins depend on their dynamic character. Allostery, in particular, relies on ligand-modulated shifts in these conformational ensembles. Hsp70s are allosteric molecular chaperones with conformational landscapes that involve large rearrangements of their two domains (viz. the nucleotide-binding domain [NBD] and substrate-binding domain [SBD]) in response to adenine nucleotides and substrates. However, it remains unclear how the Hsp70 conformational ensemble is populated at each point of the allosteric cycle and how ligands control these populations. We have mapped the conformational species present under different ligand-binding conditions throughout the allosteric cycle of the Escherichia coli Hsp70 DnaK by two complementary methods, ion-mobility mass spectrometry and double electron-electron resonance. We placed one spin label on each of the NBD and SBD, and generated the distance distribution profiles between each spin pair under the different conditions of ATP binding, ATP + ligand, and ADP + ligand. Each condition corresponds to a different step in the allosteric cycle of the E. coli Hsp70 Dnak. Based on the available structure of each domain and using triangulation, we were able to map the conformational species of the whole protein under different ligand binding conditions of the allosteric cycle of DnaK. Our results, obtained under biologically relevant ligand-bound conditions, confirm the current picture derived from NMR and crystallographic data of domain docking upon ATP binding and undocking in response to ADP and substrate. Additionally, we find that the helical lid of DnaK is a highly dynamic unit of the structure in all ligand-bound states. Importantly, we demonstrated that DnaK populates a partially docked state in the presence of ATP and substrate and that this state represents an energy minimum on the DnaK allosteric landscape. Because Hsp70s are emerging as potential drug targets for many diseases, fully mapping an allosteric landscape of a molecular chaperone like DnaK will facilitate the development of small molecules that modulate Hsp70 function via allosteric mechanisms. Funding: P41GM103521, R01EB003150 (to JHF), R01GM027616, R35GM118161 (to LMG), MRC 98101, Wellcome Trust WT008150, WT099141, and ERC IMPRESS (to CVR). Publication: A.L. Lai, E.M. Clerico, M.E. Blackburn, N.A. Patel, C.V. Robinson, P.P. Borbat, J.H. Freed, and L.M. Gierasch. J. Biol. Chem. 292, 8773-8785 (2017); PMCID: PMC5448104. |
||
|
||
|
Alex Liqi Lai (ACERT) Eugenia M. Clerico (Department of Biochemistry and Molecular Biology, University of Massachusetts, Amherst) Mandy E. Blackburn (School of Environmental, Physical, and Applied Sciences, Univeristy of Central Missouri, Warrensburg) Nisha A. Patel, Carol V. Robinson (Physical & Theoretical Chemistry Laboratory, S. Parks Rd., Oxford, UK) Peter P. Borbat, Jack H. Freed (ACERT) and Lila M. Gierasch (Department of Biochemistry and Molecular Biology, University of Massachusetts, Amherst; Department of Chemistry, University of Massachusetts, Amherst) |
||
|
|
About ACERT Contact Us |
Research |
Outreach |
ACERT is supported by grant 1R24GM146107 from the National Institute of General Medical Sciences (NIGMS), part of the National Institutes of Health. |
|||||
| ||||||||