.svg) National Institute of General Medical Sciences |
 |
 |
National Biomedical Resource for |
| ACERT's Service and Collaborative Projects | |
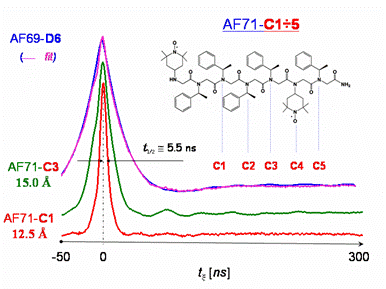
The recent development of biomimetic oligomers capable of autonomous folding has created a need for new methods to evaluate the structure and dynamics of these systems. We have found that double-quantum coherence (DQC)-ESR is very useful to characterize the folding of oligo-N-substituted glycine “peptoids.” Peptoids are amino acid analogs where the basic unit consists of N-substituted glycine derivatives. As opposed to peptides, where the side chain is bound to the alpha-carbon, in peptoid, the side chain or functional group is bonded to the alpha nitrogen. Relative to polypeptides, polypeptoids have their side chains shifted by one position along the backbone. Peptoids were designed as a new series of potentially bioactive compounds due to a variety of possible functional groups, and they have potential in drug delivery due to their expected metabolic stability. These peptoid oligomers were synthesized by solid phase techniques incorporating the spin label, TEMPO as a side chain. A series of doubly spin-labeled oligomers were prepared in which the relative positions of the TEMPO groups along the backbone was systematically varied as shown in the Figure below. The DQC-ESR signals are also shown in the Figure. They correspond to relatively short distances over the range of 12-18 Å with broad distributions, reflecting the flexibility of the peptoids. These results thus demonstrate the capability of DQC-ESR to provide accurate distances and distributions, in this important shorter distance range, previously accessible only by cw-ESR in a more indirect fashion. The very rapid decay of the DQC-ESR signals, due to the strong dipolar coupling, required the nanosecond time resolution available at ACERT. Thus, the use of DQC-ESR will facilitate the study of increasingly complex peptoid structures as the capabilities for design of folded heteropolymers continue to advance. Publication: A.T. Fafarman, P.P. Borbat, J.H. Freed, and K. Kirschenbaum, Chem. Commun., (2007), 377-379; not indexed in PubMed or PubMed Central, no vol. # |
|
 |
|
|
Fafarman, K. Kirshenbaum (Dept. Of Chemistry, NYU, New York) P.P. Borbat, J.H. Freed (ACERT) |
|
|
|
About ACERT Contact Us |
Research |
Outreach |
ACERT is supported by grant 1R24GM146107 from the National Institute of General Medical Sciences (NIGMS), part of the National Institutes of Health. |
|||||
| ||||||||