.svg) National Institute of General Medical Sciences |
 |
 |
National Biomedical Resource for |
| ACERT's Service and Collaborative Projects | |
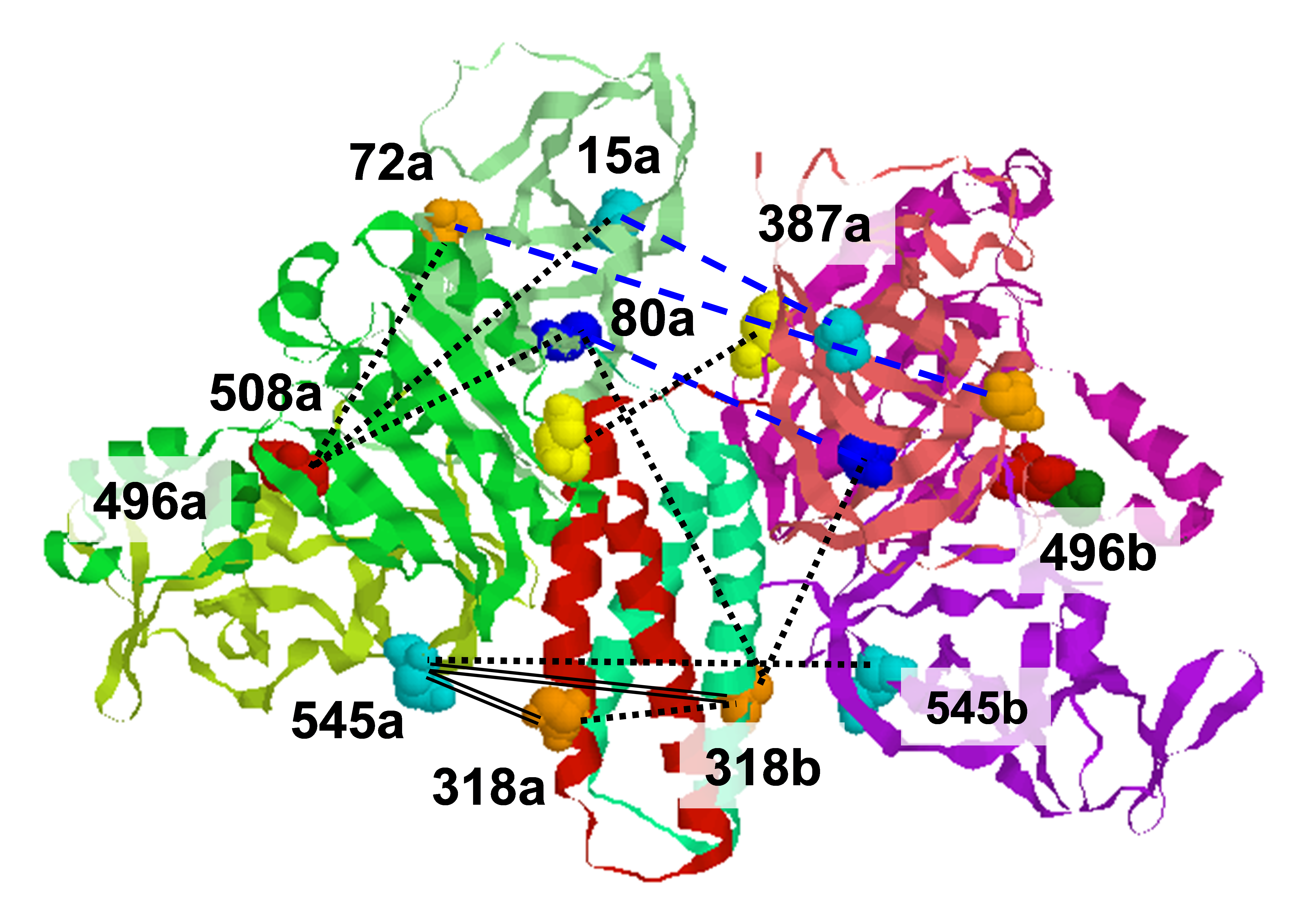
How chemoreceptors regulate the activity of the histidine kinase CheA is a central question in understanding the signaling pathway that controls bacterial chemotaxis. The interactions between receptors and CheA require the involvement of the coupling protein CheW. We have applied the pulsed ESR techniques of DQC and DEER to measure distances between nitroxide spin-labels in these proteins. Site-directed cysteine mutations of CheA and CheW allow the placement of spin-labels at specific positions on the individual domains, and the associations can then be mapped by multiple distance measurements. From the reported crystal structure of T. maritima CheA Δ289 (P3-P4-P5 domain) and solution structure of T. maritima CheW, obtained by Crane and co-workers, we chose several residues in the two proteins that cover a wide range of distances and are exposed to the solvent. After mutating the residues to cysteine, the expressed proteins were purified and reacted with nitroxide spin label. Since the CheA/CheW complex is a dimer it contains four nitroxides. This necessitated careful selection of labeling sites so only the desired distance is dominant in the experiment. The structure was inferred from the DQC/DEER data by metric matrix distance geometry. It is quite unexpected, since it was widely believed that CheW binds on the opposite side of P5 in CheA where residues 568 and 579 are located. This new structure obtained by ESR soon was supported by X-ray data on the structure of the P4/P5 fragment complexed with CheW. The unit cell contains two P4/P5/CheW in vastly different conformations, but the structure of CheW/P5 is essentially the same and very close to what first emerged from pulsed ESR, consistent with very strong binding Kd=50nM. In the CheA/CheW complex Kd=5nM, even though the structure of CheW/P5 is very close to that in P4/P5/CheW complex, suggesting P3 involvement, which may also be studied by ESR. This work sets the stage for the routine use of pulsed ESR for ternary and quaternary structure determination. Publication: S.-Y. Park, P.P. Borbat, G. Gonzalez-Bonet, J. Bhatnagar, A.M. Pollard, J.H. Freed, A.M. Bilwes, and B.R. Crane, Nat. Struct. Mol. Biol., 13, 400-407 (2006); no PMCID |
|
|
|
|
S.-Y. Park, B. R. Crane (Dept. of Chemistry and Chemical Biology, Cornell University) P. P. Borbat, A. M. Bilwes, J. H. Freed |
|
|
|
About ACERT Contact Us |
Research |
Outreach |
ACERT is supported by grant 1R24GM146107 from the National Institute of General Medical Sciences (NIGMS), part of the National Institutes of Health. |
|||||
| ||||||||