.svg) National Institute of General Medical Sciences |
 |
 |
National Biomedical Resource for |
| ACERT's Service and Collaborative Projects | ||
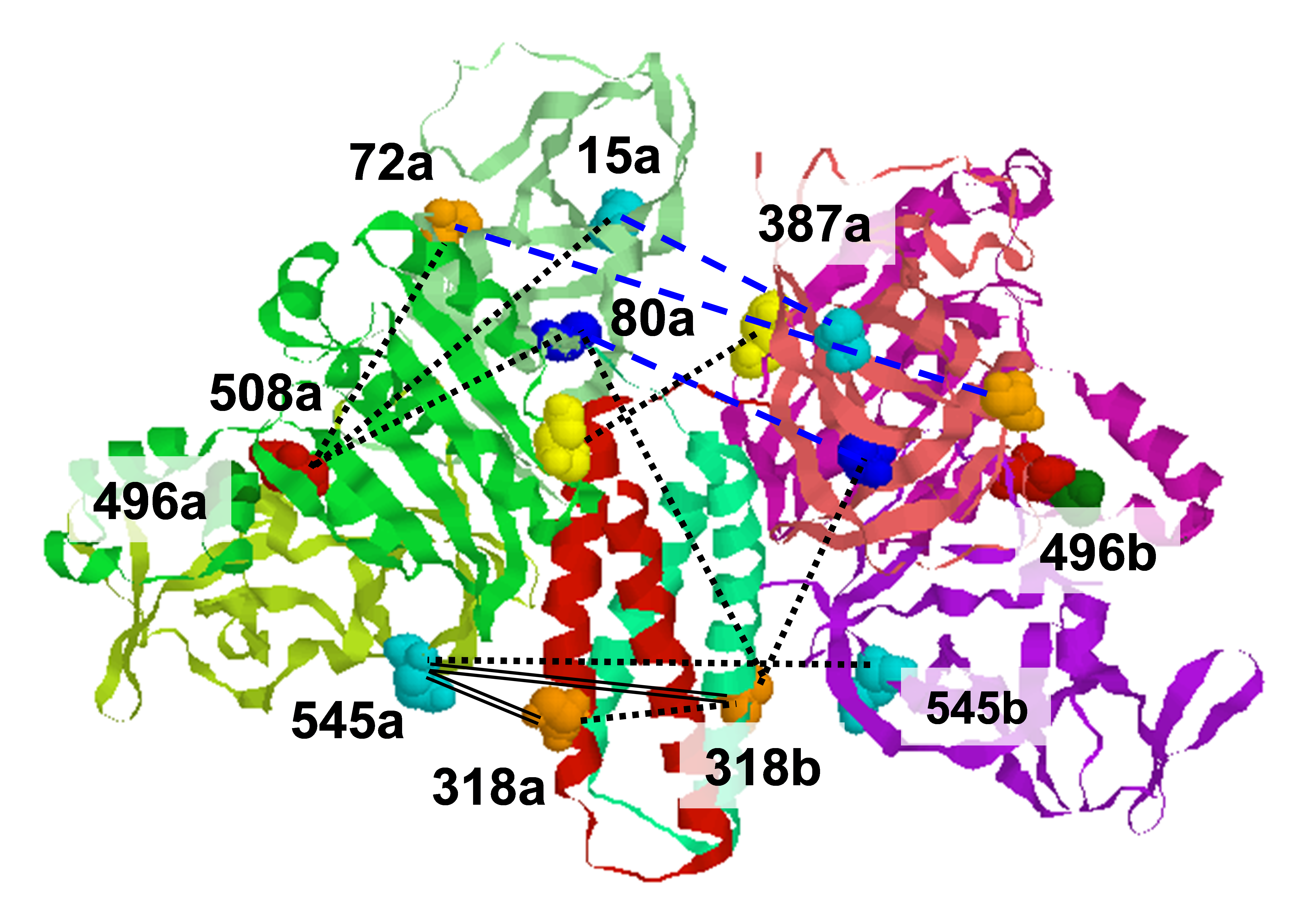
ESR studies of nitroxide spin labels have become a valuable tool for the investigation of protein structure and dynamics. The spectra reflect mobility and chemical environment of the spin label. Different motions, covering a wide range of amplitudes and different timescales, are simultaneously present: they comprise overall protein tumbling and refolding processes, backbone fluctuations, and side-chain isomerizations. Recognition of the motion of the spin label about its tether, even though it is not the objective of the investigation, is a prerequisite for extracting useful information. We have developed a theoretical approach to analyze the conformations and dynamics of the side-chain of the MTSSL spin-label attached to a model alpha-helix in order to identify general features, which can be useful for the interpretation of ESR spectra of spin-labeled proteins. Torsional energy profiles have been obtained by quantum mechanical methods taking into account the constraints imposed by the local environment. This enables a description of the system in terms of a limited number of rotamers, undergoing conformational jumps and librations about the minima of the side-chain torsional potential. A diffusive treatment of the dynamics is used which takes account of the energetic and frictional features of the flexible tail. This yields estimates of the amplitude and time-scale of the chain motions of the tether. Rotations around the chi1, chi2, and chi3 bonds (see figure) are very unlikely, and only the chi 4 and chi 5 conformational transitions (and torsional oscillations) affect the ESR spectrum. The potential energy graphs for motion about these bonds are illustrated below. The results of this analysis have been incorporated into an approximate form of the stochastic Liouville equation, that is reasonable for 9 GHz ESR spectra. With virtually all the parameters determined independently, the 9 GHz spectra from T4 Lysozyme spin-labeled at the 72 residue are well fit by this theory. For the methyl substituted MTSSL spin label, only a few non-interconverting conformers are possible whose mobility is limited to torsional oscillations, yielding almost identical spectra, typical of slightly mobile nitroxides. The standard MTSSL spin label yields more complex spectra which result from the simultaneous presence of constrained and mobile chain conformers. The agreement between experiment and theory for this case is illustrated below. Publication: F. Tombolato, A. Ferrarini, and J.H. Freed, J. Phys. Chem. B, 110, 26248-26259 (2006); PMC2883179 |
||
|
||
|
Fabio Tombolato, Alberto Ferrarini (Universita di Padova, Italy) J. H. Freed (ACERT) |
||
|
|
About ACERT Contact Us |
Research |
Outreach |
ACERT is supported by grant 1R24GM146107 from the National Institute of General Medical Sciences (NIGMS), part of the National Institutes of Health. |
|||||
| ||||||||