.svg) National Institute of General Medical Sciences |
 |
 |
National Biomedical Resource for |
| ACERT's Service and Collaborative Projects | ||
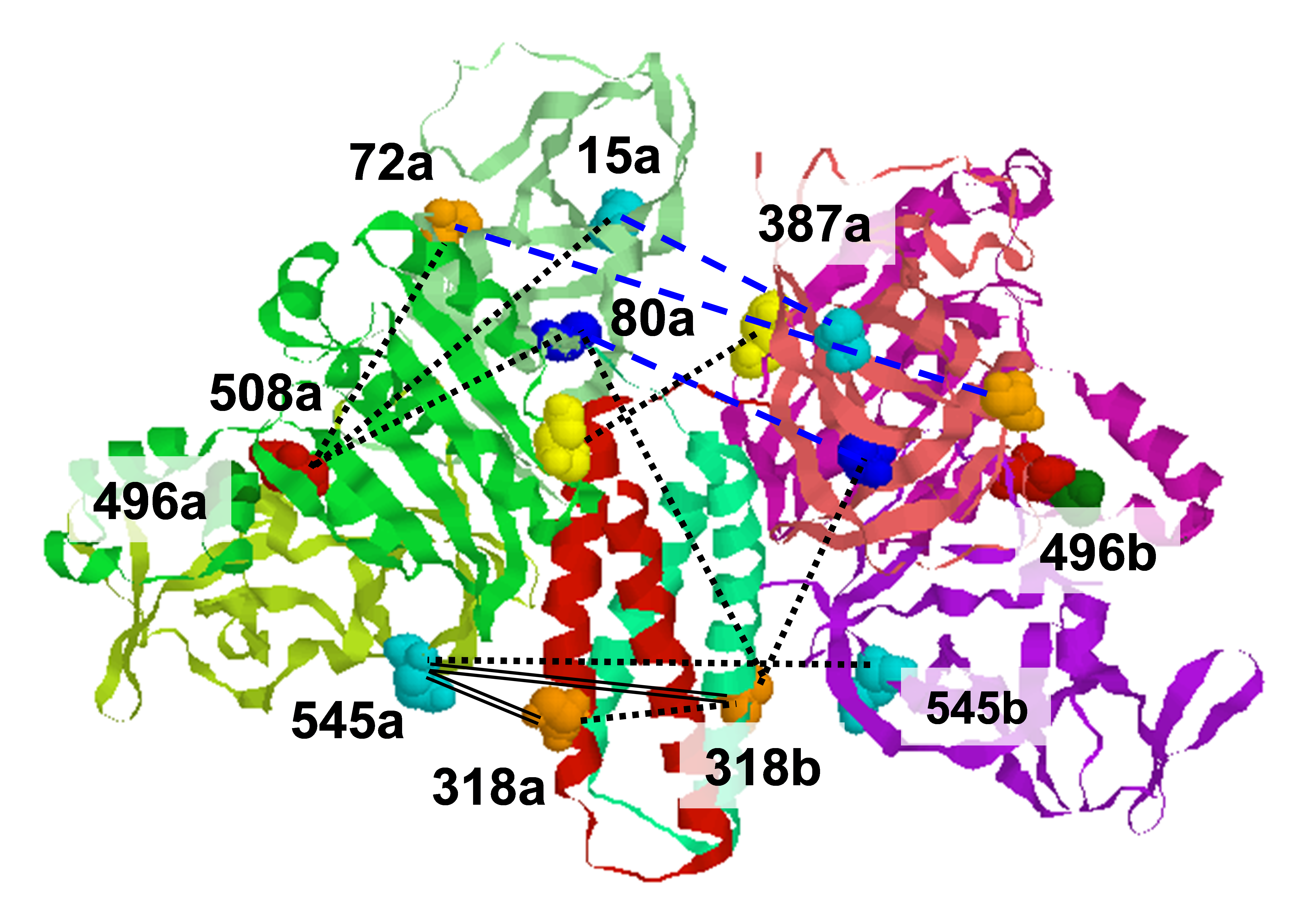
Diphthamide is a posttranslationally modified His residue in eukaryotic translation elongation factor 2 (EF-2), a GTPase required for the translocation step of ribosomal protein synthesis. The name "diphthamide" comes from diphtheria toxin, which specifically ADP-ribosylates this posttranslational modification, inhibits protein synthesis and leads to host cell death. Understanding how diphthamide is synthesized and how the biosynthesis is regulated will provide useful insights into its biological function. One of the most intriguing facts about diphthamide is that Dph1, one of the genes involved in the biosynthesis, is found to be a tumor suppressor gene in humans. Loss of a Dph1 gene is observed in 80% of ovarian cancers as well as in other types of cancers, such as breast, lung and brain malignancies. Here we present structural and biochemical evidence showing that the first step of diphthamide biosynthesis in archaea uses a novel iron-sulfur cluster enzyme, Dph2. Dph2 is a homodimer and each monomer contains a [4Fe-4S] cluster. Biochemical data suggest that unlike known radical SAM enzymes, Dph2 does not form 5´-deoxyadenosyl radicals. Instead, it breaks the other C-S bond of SAM and transfers the 3-amino-3-carboxylpropyl group to EF-2, possibly via a radical mechanism. This work suggests that archaeal Dph2 represents a novel radical SAM enzyme with a three-dimensional structure different from known radical SAM enzymes and catalyzes unprecedented chemistry. In this study ESR spectroscopy provided important evidence for the existence of the iron-sulfur cluster and changes in its oxidation state. PhDph2 as purified has essentially no EPR signal, indicative of a [4Fe-4S]2+ state. Upon reduction with dithionite, the protein has a strong EPR signal. The g- and the temperature-dependence are typical of a [4Fe-4S] ]+ cluster. Quantification of the EPR spectrum suggests the presence of 0.3 [4Fe-4S] ]+ per PhDph2: Publication: Y. Zhang, X. Zhu, A. Torelli, M. Lee, B. Dzikovski, R.M. Koralewski, E. Wang, J.H. Freed, C. Krebs, S.E. Ealick, and H. Lin, Nature, 465, 891-896 (2010); PMC3006227 |
||
|
||
|
Yang Zhang, Xuling Zhu, Andrew Torelli (Department of Chemistry and Chemical Biology, Cornell University) Michael Lee (Department of Biochemistry and Molecular Biology, Pennsylvania State University) Boris Dzikovski, Rachel M. Koralewski, Eileen Wang, Jack H. Freed (Department of Chemistry and Chemical Biology, Cornell University) Carsten Krebs (Department of Biochemistry and Molecular Biology and Department of Chemistry, Pennsylvania State University) Steven E. Ealick, Hening Lin (Department of Chemistry and Chemical Biology, Cornell University) |
||
|
|
About ACERT Contact Us |
Research |
Outreach |
ACERT is supported by grant 1R24GM146107 from the National Institute of General Medical Sciences (NIGMS), part of the National Institutes of Health. |
|||||
| ||||||||