.svg) National Institute of General Medical Sciences |
 |
 |
National Biomedical Resource for |
| ACERT's Service and Collaborative Projects | |
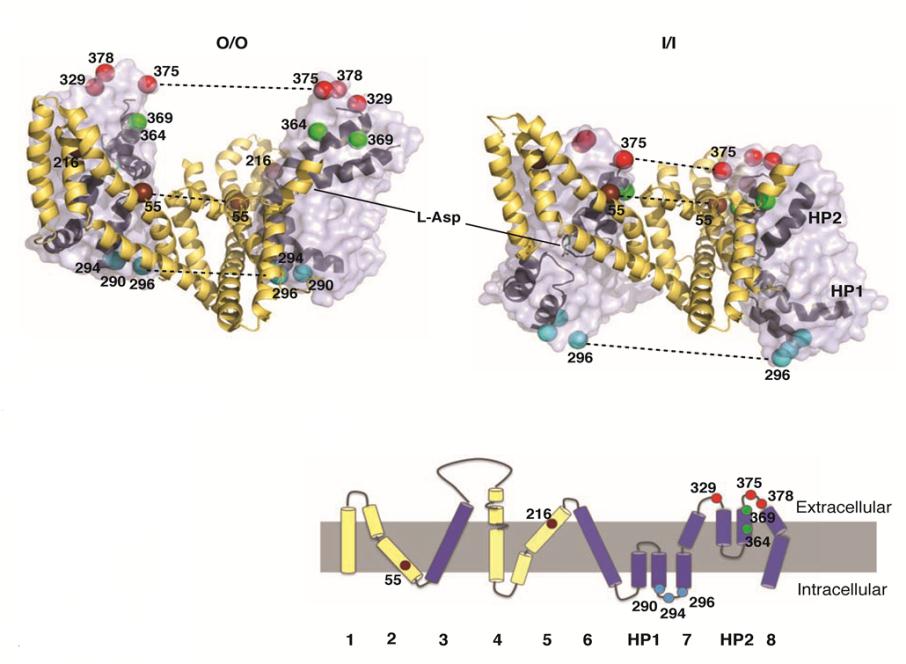
Understanding the mechanism of function and inhibition of glutamate transporters is of high biomedical importance, since glutamate, which is a major excitatory transmitter in the central neural system, plays a key role in physiological processes such as normal brain function including learning, memory formation, and cognition. Impairment of glutamate transport is implicated in cerebral ischemia, neurotoxicity and a number of other neurological diseases and ailments. Glutamate transporters residing in the plasma membranes of glial cells and neurons are primarily responsible for the transmitter uptake from the extracellular space and for tight regulation of its concentration. We studied by pulsed dipolar ESR spectroscopy (PDS) the structural rearrangements occurring during the transport cycle of an archaeal homologue of mammalian glutamate transporters, GltPh, a sodium-aspartate symporter from Pyrococcus horikoshii. GltPh is a homotrimer, similarly to mammalian glutamate transporters. Here we show structural and functional information about this transporter in solution and, importantly, in natural lipid environments. The high quality of our PDS data, made possible by the excellent sensitivity of the PDS spectrometer at ACERT, allowed us to carry out a detailed data analysis for a number of key spin-labeled sites and thereby to quantify the population of protomers in different conformations. Our analysis shows that GltPh protomer attains the outward or inward facing conformation at random. This is clear spectroscopic evidence that GltPh protomer functions independently. Our analysis also shows that these conformational states are almost equally populated at all conditions studied, indicating that they are nearly equienergetic. Publication: E. Georgieva, P.P. Borbat, C. Ginter, J.H. Freed, and O. Boudker, Nat. Struct. Mol. Biol., 20, 215-221 (2013); PMC3565060. |
|
 |
|
|
Elka R. Georgieva, Peter P. Borbat (ACERT) Chris Ginter , Olga Boudker (Dept. of Physiology and Biophysics, Weill Cornell Medical College, New York, NY) Jack H. Freed (ACERT) |
|
|
|
About ACERT Contact Us |
Research |
Outreach |
ACERT is supported by grant 1R24GM146107 from the National Institute of General Medical Sciences (NIGMS), part of the National Institutes of Health. |
|||||
| ||||||||