.svg) National Institute of General Medical Sciences |
 |
 |
National Biomedical Resource for |
| ACERT's Service and Collaborative Projects | |
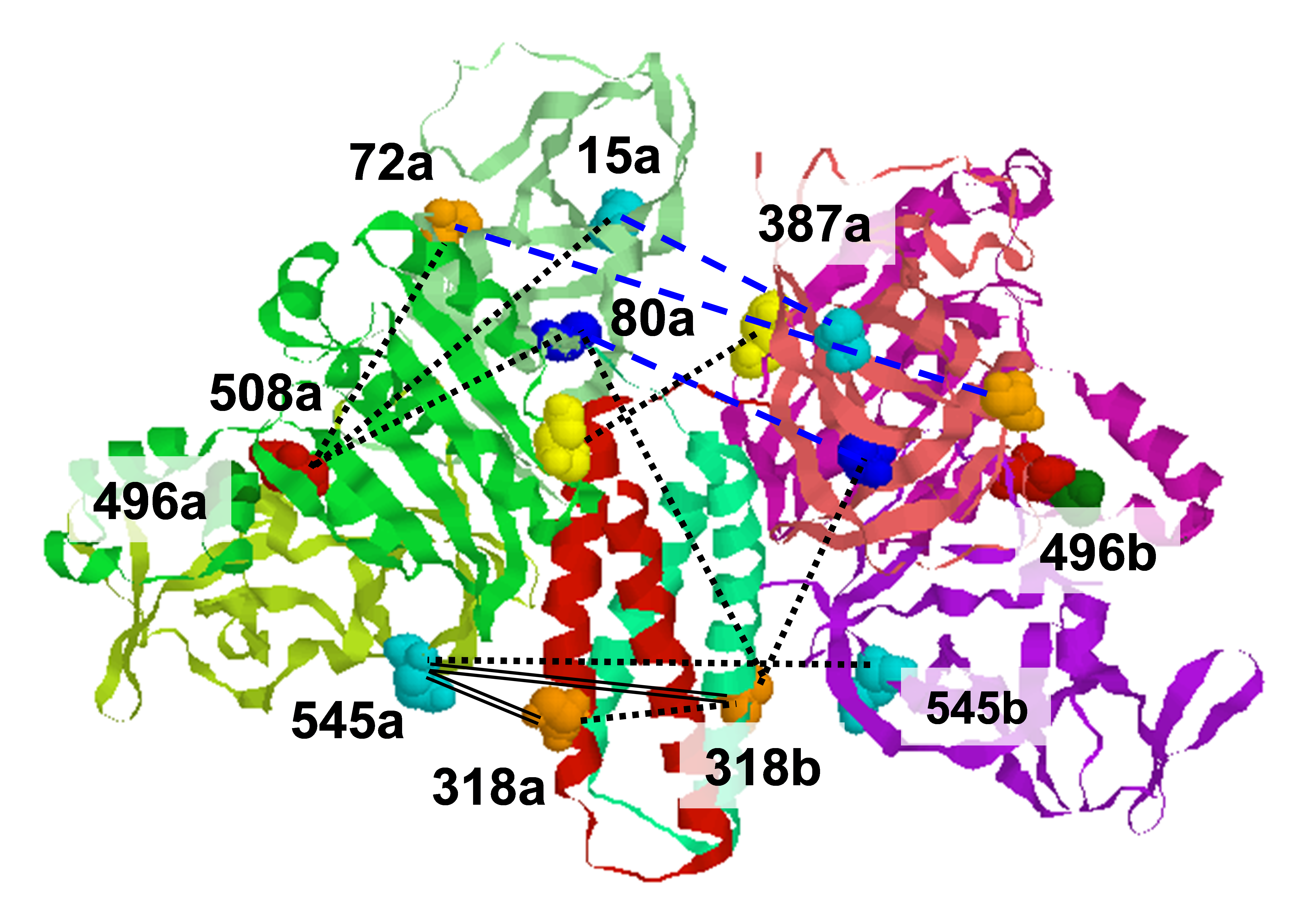
Chemotaxis is a process that many flagellated bacteria use to search out the suitable place for its survival. Inner membrane chemoreceptors, one of the components in chemotaxis, sense specific sets of chemicals in the environment through the periplasmic domain, send a signal to the cytoplasmic components which ultimately leads the cell either to swim in the same direction (in case of beneficial chemical sensing) or to change the swimming direction and leave the place (in case of harmful chemical sensing). The chemoreceptor is a homodimer with each monomer having a modular architecture. Each monomer is constructed with a ligand sensing, N-terminal periplasmic domain, flanked by two transmembrane helices, a cytoplasmic kinase control domain that interacts with other cytoplasmic components involved in the process, and a HAMP domain (Histidine kinases, Adenylyl cyclases, Methyl accepting chemotaxis proteins, and Phosphatases) bridging the periplasmic and kinase control domain. If the chemoreceptor senses repellent (attractant) it sends a signal to the kinase control domain which leads to clockwise (counter-clockwise) rotational bias of the flagella reversing (keeping) in the swimming direction of the cell. The HAMP domain, being present between the periplasmic and kinase control domain, mediates the transduction of the signal from the periplasmic domain to the kinase control domain and plays a crucial part in the process. We constructed two soluble chemoreceptor chimeras, HAMP1-Tar and HAMP1-2-Tar, with repellent binding and attractant binding phenotype respectively. We mutated and spin-labeled different sites in the HAMP domain in the two constructs, and studied the structure with DEER spectroscopy. We found that the distance distribution between the pairs of cysteines in HAMP1-Tar (repellent binding phenotype) was much broader than that between the respective pairs of cysteines in HAMP1-2-Tar (attractant binding phenotype). The distance distribution between the cysteine pairs in HAMP1-2-Tar I88G mutant we found that the distribution was much broader than that in the HAMP1-2-Tar construct and resembles with the distribution in the HAMP1-Tar construct. We conclude that ordered HAMP domain sends out a phosphorylation cascade deactivating signal, whereas disordered HAMP domain sends out an activating signal. We can also extrapolate our conclusion to understand the signal transduction in the chemoreceptor. When the chemoreceptor binds an attractant in the periplasmic domain, the HAMP domain switches to an ordered conformation sending out the deactivating signal and in case of repellent binding the HAMP domain switches to the disordered state sending out the activating signal for the cytoplasmic phosphorylation cascade. Publication: M.V. Airola, N. Sukomon, D. Samanta, P.P. Borbat, J.H. Freed, K.J. Watts, and B.R. Crane, PLoS Biol., 11, 1-13, e1001479 (2013); PMC3570549. |
|
|
|
|
Dipanjan Samanta, Michael V. Airola, Nattakan Sukomon (Department of Chemistry and Chemical Biology, Cornell University) Peter P. Borbat, Jack H. Freed (ACERT) and Brian R. Crane (Department of Chemistry and Chemical Biology, Cornell University) |
|
|
|
About ACERT Contact Us |
Research |
Outreach |
ACERT is supported by grant 1R24GM146107 from the National Institute of General Medical Sciences (NIGMS), part of the National Institutes of Health. |
|||||
| ||||||||