.svg) National Institute of General Medical Sciences |
 |
 |
National Biomedical Resource for |
| Pulsed ESR: Distance Measurements |
|
Site-directed nitroxide spin labeling opens doors for use of ESR in structure determination and the study of functions of a broad class of biomolecules such as proteins and RNA. One method uses higher order coherences which can be created and manipulated in systems of coupled electron spins. Double-quantum coherence (DQC) between two electron spins coupled by their dipole-dipole interaction is of particular interest to us, since this coherence provides the tool for separating weak dipolar couplings from stronger interactions for accurate measurements of distances over a broad range, 10Å…..40-80Å, depending on the system and conditions. Let’s consider two interacting spins a and b. Possible coherences are: |
||||||
|
||||||
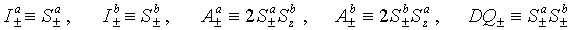 |
||||||
All these coherences can be manipulated by pulses and be refocused. Refocusing of DQ± is particularly useful, because it singles out the part of the signal that evolves solely due to spin coupling. |
||||||
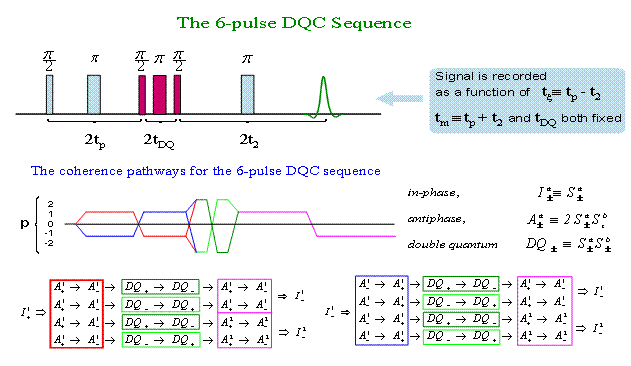 |
||||||
Once we have a bilabel rigid molecular structure, the workflow for the 6-pulse DQC sequence will look as follows: |
||||||
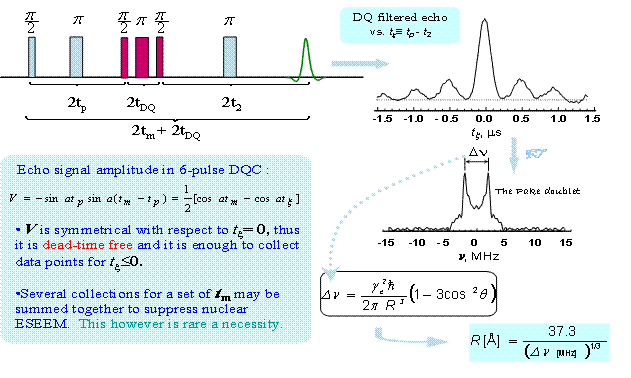 |
||||||
Our overall aim in this area is to fundamentally advance our knowledge of structure and functions of biomolecules and to increase the experimental throughput of protein structure determinations using a small number of longer distance constraints, provided by modern techniques of site-directed spin labeling and pulsed ESR distance measurement techniques, and to further refine these techniques. Additional:
|
|
© 2022 |
|
About ACERT Contact Us |
Research |
Outreach |
ACERT is supported by grant 1R24GM146107 from the National Institute of General Medical Sciences (NIGMS), part of the National Institutes of Health. |
|||||
| ||||||||