|
Dipolar Spectroscopy – Single-Resonance Methods
P. P. Borbat and J. H. Freed.
eMagRes 6 425-462 (2017)
<doi: 10.1002/9780470034590.emrstm1519>
PMID: [None - Book] PMCID:
[None - Book]
|
|
|
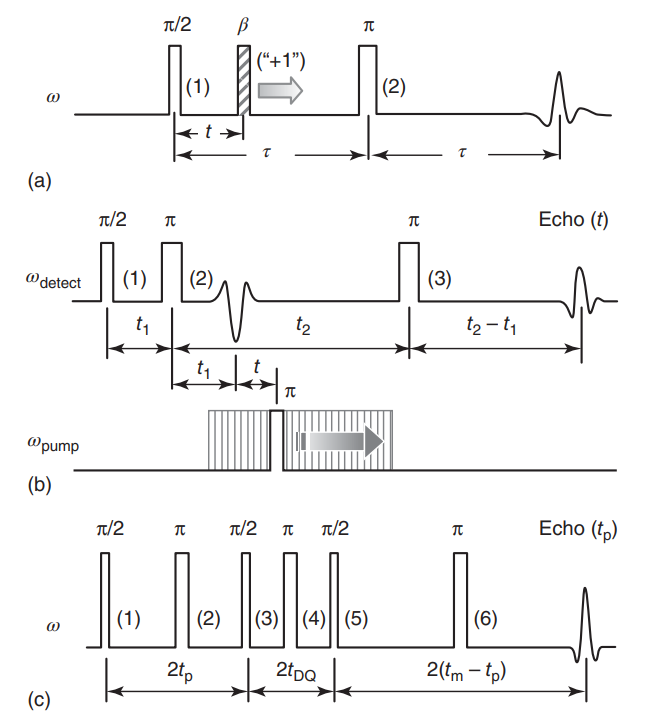
ABSTRACT: A range of powerful techniques based on pulse EPR spectroscopies is used extensively for measuring electron spin dipolar couplings between native or introduced paramagnetic reporters, from which accurate distances, and in certain cases molecular orientations, can be determined to aid in solving biomolecular structures. Notably, several versatile pulse EPR methods of high sensitivity were developed over the past two decades. The two main groups of pulse methods are represented by single- and double-resonance techniques, which together constitute pulse dipolar EPR spectroscopy (PDS). We describe the first group by outlining the basic principles and illustrate key techniques with several examples from the literature, as well as some newly produced for purposes of this chapter. Specifically, we describe double-quantum coherence (DQC) methods for robust selection of the dipolar coupling by filtering out undesired spin coherence pathways via phase cycling, and we also discuss single-resonance methods such as four- and five-pulse single-quantum coherence (SQC) methods, all of which benefit from maximizing the spectral coverage. The common features and conceptual differences between single- and double-resonance methods are discussed. In addition, the extensions of modern DQC and SQC methods to two-dimensional correlation spectroscopy for eliciting orientational information are included. Other types of 'single-frequency' PDS methods, not all of which are based on principles behind fully coherent single-resonance methods, are also discussed to show the diversity of PDS methods.
|
|
|
Pulse Dipolar Electron Spin Resonance: Distance Measurements
P.P. Borbat and J.H. Freed.
In Structural Information from Spin-Labels and Intrinsic Paramagnetic Centers in the Biosciences. Structure and Bonding. Vol. 152. J. Harmer and C. Timmel, Eds.
Springer: Heidelberg, Germany; New York, USA, 2013; pp. 1-82.
|

|
|
Pulse Dipolar Electron Spin Resonance: Distance Measurements
P.P. Borbat and J.H. Freed.
In Structural Information from Spin-Labels and Intrinsic Paramagnetic Centers in the Biosciences. Structure and Bonding. Vol. 152. J. Harmer and C. Timmel, Eds.
Springer: Heidelberg, Germany; New York, USA, 2013; pp. 1-82.
<doi: 10.1007/430_2012_82>
PMID: [None - Book] PMCID:
[None - Book]
|

|
|
ABSTRACT: In recent years electron spin resonance (ESR) has provided the means to obtain structural constraints in the field of structural biology on the nanoscale by measuring distances between paramagnetic species, which usually have been nitroxide spin-labels. These ESR methods enable the measurement of distances over the wide range from ca. 6–10 Å to nearly 90 Å. While cw methods may be used for the shortest distances, it is the pulse methods that enable this wide range, as well as determination of the distributions in distance. In this chapter we first describe the underlying theoretical concepts for understanding the principal pulse methods of double quantum coherence (DQC)-ESR and double-electron–electron-resonance (DEER), which we collectively refer to as Pulse-Dipolar ESR Spectroscopies (PDS). We then provide technical aspects of pulse ESR spectrometers required for high quality PDS studies. This is followed by an extensive description of sensitivity considerations in PDS, based largely upon our highly sensitive 17.3 GHz pulse spectrometer at ACERT. This description also includes a comparison of the effectiveness of the respective PDS pulse methods. In addition, the newer methods of 5-pulse DEER, which enables longer distances to be measured than by standard DEER, and 2D-DQC, which provides a convenient mapping for studying orientational coherence between spin labels and their interspin vector, are described.
|
|
|
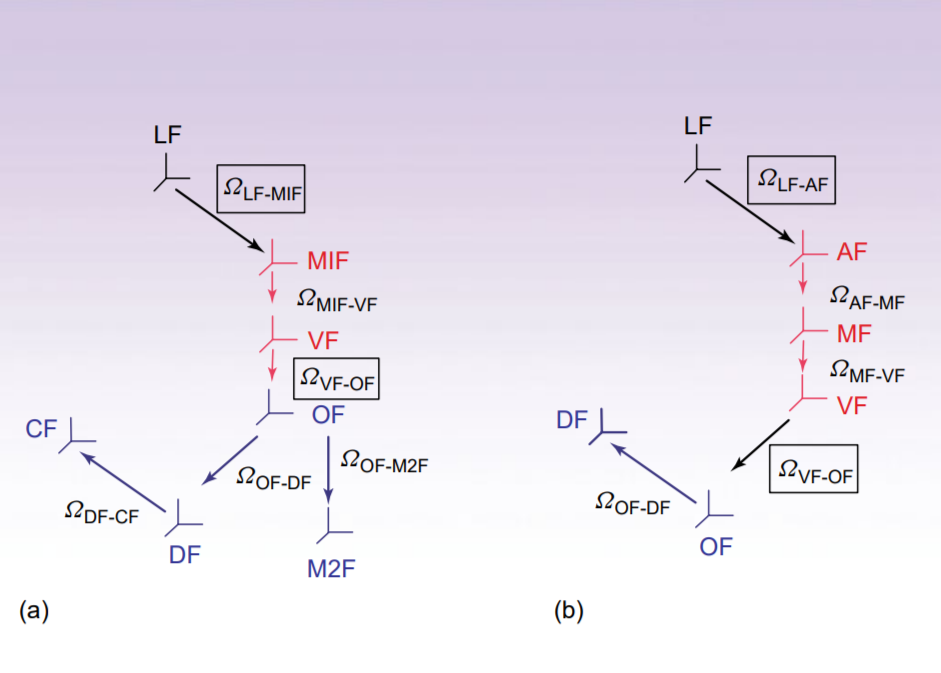
Protein Dynamics by NMR Spin Relaxation: The Slowly Relaxing Local Structure Perspective
E. Meirovitch, A. Polimeno, and J.H. Freed.
In E. Mag. Res. Online, R.K. Harris and R.L. Wasylishen, Eds.
John Wiley & Sons, Inc.: New York, 2011; pp 1-9.
<doi: 10.1002/9780470034590.emrstm1243>
PMID: [None - Book] PMCID:
[None - Book]
|
|
|
ABSTRACT: The two-body (protein and probe) coupled rotator slowly relaxing local structure (SRLS) approach, implemented for NMR spin relaxation in proteins, is presented. SRLS is based on a Smoluchowki equation that includes the tensorial descriptions of the diffusing rotators and (via the local ordering) their dynamical coupling. This yields spectral densities that enter the expressions for the experimentally measured relaxation parameters. The constrained motion of the probe is treated by analogy with the standard treatments of restricted motions in liquids. Such restricted motions are largely recovered in the limit of large time scale separation between the rotators, i.e., when mode-coupling is not important. We found that the tensorial properties are not simple for proteins even in this limit. Their complexity has to be accounted for to obtain physically insightful pictures of protein dynamics. The traditional method for analyzing NMR spin relaxation in proteins is model-free (MF). This method treats only the simplest tensorial properties and implies large time-scale separation. Only when these conditions are fulfilled the analytical MF spectral densities, which are limiting cases of SRLS, may be used. Since they are not fulfilled in most actual cases, the MF-based pictures of protein dynamics are unreliable.
|
|
|
Stochastic Methods for Magnetic Resonance Spectroscopies
A. Polimeno, V. Barone, and J.H. Freed.
In Computational Strategies for Spectroscopy: From Small Molecules to Nano Systems; V. Barone, Ed.
Wiley: New York, NY, 2012; Chapter 12.
<doi: 10.1002/9781118008720.ch12>
PMID: [None - Book] PMCID:
[None - Book]
|

|
|
ABSTRACT: Physicochemical properties of molecules in solution depend on the action of different motions at several time and length scales, and information on multiscale dynamics can be gained, in principle, by a variety of spectroscopic techniques. In this work we review theoretical tools for the investigation of "slow" molecular motions, such as solvent cage effects in liquids and liquid crystals, global and local dynamics in proteins, reorientation dynamics, and internal (conformational) degrees of freedom. Spectroscopic techniques which are most sensitive to such motions are electron spin resonance and nuclear magnetic resonance and they require ad hoc theoretical treatment. In particular, we discuss the definition of multidimensional stochastic models and their treatment to interpret magnetic resonance spectroscopic data of rigid and flexible molecules in isotropic media, liquid crystals, and biosystems.
|
|
|
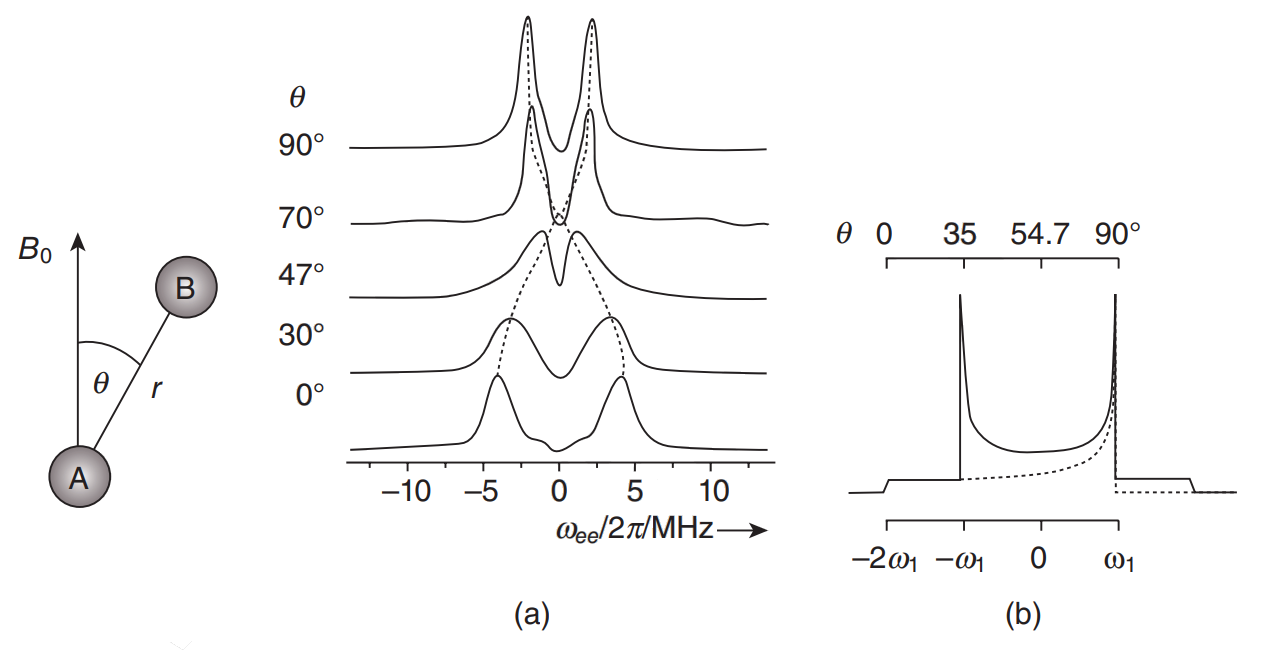
Distance Measurements: Continuous-Wave (CW)- and Pulsed Dipolar EPR
S.K. Misra and J.H. Freed.
In Multifrequency Electron Paramagnetic Resonance, S.K. Misra, Ed.
Wiley-VCH: New York, 2011; Chapter 12, pp. 545-588.
<doi: 10.1002/9783527633531.ch12>
PMID: [None - Book] PMCID:
[None - Book]
|
|
|
INTRODUCTION: Information on long-range distances between selected sites is a prerequisite to understanding the detailed structure of complex systems. In NMR utilizing a spin label, it is possible to measure distances in the range between 8 Å to, at most, 25 Å. On the other hand, with EPR, distances in the range between about 10 and 90 Å can and have been measured. EPR spectroscopy may be the best practical technique for complex systems, since methods such as small-angle scattering, X-ray scattering and small-angle neutron scattering have insufficient contrast whereas fluorescence resonant energy transfer (FRET) lacks key virtues of EPR noted below. Distance measurements in EPR are based on exploitation of the dipolar interaction (DI), that depends on the distance between two paramagnetic probes, and which may be identical or may differ from each other, and are located at different spatial positions in the sample. They can be introduced using site-directed mutagenesis (also known as site-directed spin labeling; SDSL), a technique which enables the investigation of, for example, proteins and other biomolecules. In fact, the signature of the DI can be clearly seen in CW-EPR spectra under favorable circumstances for short distances. On the other hand, in pulsed EPR [hereafter referred to as pulsed dipolar spectroscopy; PDS), experiments can be designed specifically to clearly distinguish the dipolar interaction. The two commonly used PDS techniques are: (i) pulsed electron double resonance (PELDOR; this term was originally coined by the Russians, who first developed the technique), which is also referred to as double electron-electron resonance (DEER; this term was introduced later in the USA, and will be used hereafter in this chapter); and (ii) double quantum coherence (DQC)-EPR.
|
|
|
Molecular Motions
S.K. Misra and J.H. Freed.
In Multifrequency Electron Paramagnetic Resonance, S.K. Misra, Ed.
Wiley-VCH: New York, 2011; Chapter 11, pp. 497-544.
|

|
|
Molecular Motions
S.K. Misra and J.H. Freed.
In Multifrequency Electron Paramagnetic Resonance, S.K. Misra, Ed.
Wiley-VCH: New York, 2011; Chapter 11, pp. 497-544.
<doi: 10.1002/9783527633531.ch11>
PMID: [None - Book] PMCID:
[None - Book]
|

|
|
INTRODUCTION: In this chapter we will discuss the study of molecular motion by EPR, using both continuous-wave (CW) and pulsed EPR, in particular two-dimensional electron–electron double resonance (2-D-ELDOR). In recent years, the study of protein dynamics using site-directed spin labels (SDSL) by EPR has become an important subject.
|
|
|
Structural Dynamics of Bio-Macromolecules by NMR: The Slowly Relaxing Local Structure Approach
E. Meirovitch, Y.E. Shapiro, A. Polimeno, J.H. Freed.
Progress in NMR Spectroscopy, 56, 360-405 (2010).
<doi: 10.1016/j.pnmrs.2010.03.002>
PMID:
20625480
PMCID:
PMC2899824
|

|
|
INTRODUCTION: Protein dynamics by NMR has been reviewed extensively in recent years. These surveys show decisively that information on structure should be complemented by information on motion both to properly characterize the protein, and to understand its function. The time scale accessible by NMR extends from picoseconds to days, with different methods accessing different parts of this time axis. Here we focus on heteronuclear NMR spin relaxation used to study ps to ns protein dynamics. The slow limit of this time regime is determined by the global tumbling of the protein, with the rates for internal motion of the probe being typically faster.
|
|
|
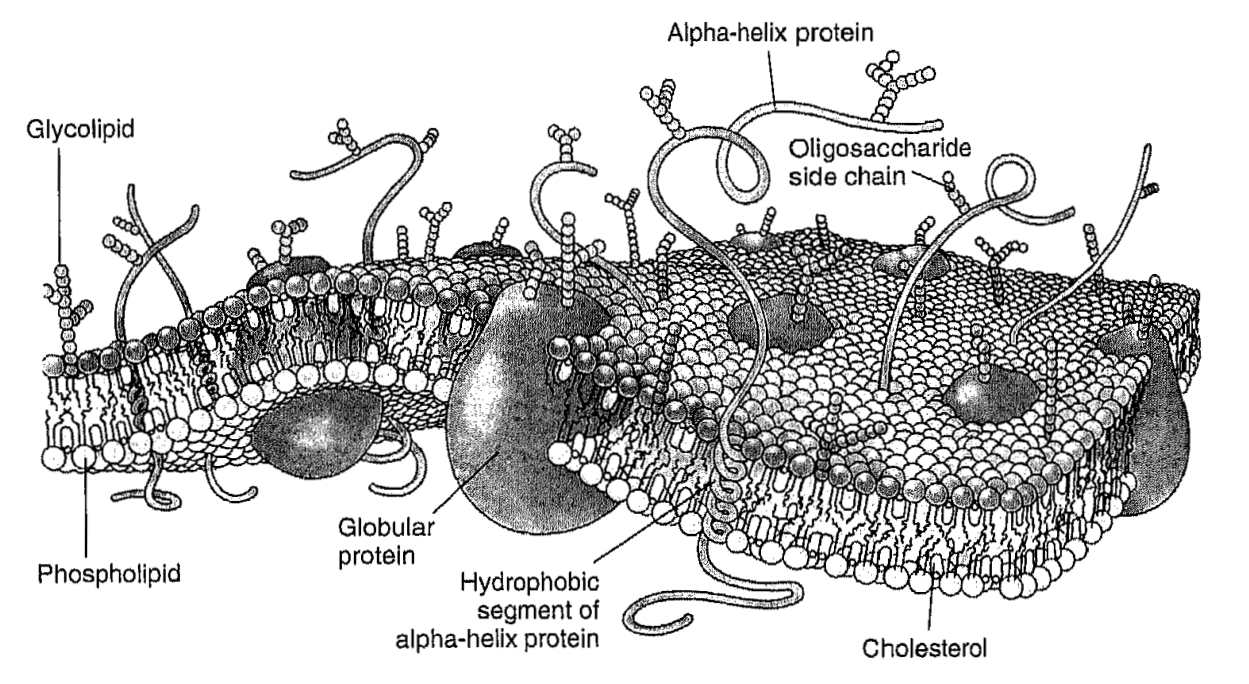
Membrane Fluidity
B. Dzikovski and J.H. Freed.
In Wiley Encyclopedia of Chemical Biology; T. P. Begley and B. A. Baird, Eds.
Wiley; Hoboken, NJ, USA, 2009; Chapter 2, pp 728-741.
|
|
|
Membrane Fluidity
B. Dzikovski and J.H. Freed.
In Wiley Encyclopedia of Chemical Biology; T. P. Begley and B. A. Baird, Eds.
Wiley; Hoboken, NJ, USA, 2009; Chapter 2, pp 728-741.
<doi: 10.1002/9780470048672.wecb313>
PMID: [None - Book] PMCID:
[None - Book]
|

|
|
ABSTRACT: In the popular fluid mosaic model for biomembranes, membrane proteins and other membrane-embedded molecules are in a two-dimensional fluid formed by the phospholipids. Such a fluid state allows free motion of constituents within the membrane bilayer and is extremely important for membrane function. The term "membrane fluidity" is a general concept, which refers to the ease of motion for molecules in the highly anisotropic membrane environment. We give a brief description of physical parameters associated with membrane fluidity, such as rotational and translational diffusion rates, order parameters, etc., and review physical methods used for their determination. We also show limitations of the fluid mosaic model and discuss recent developments in membrane science that pertain to fluidity, such as evidence for compartmentalization of the biomembrane by the cell cytoskeleton.
|
|
|
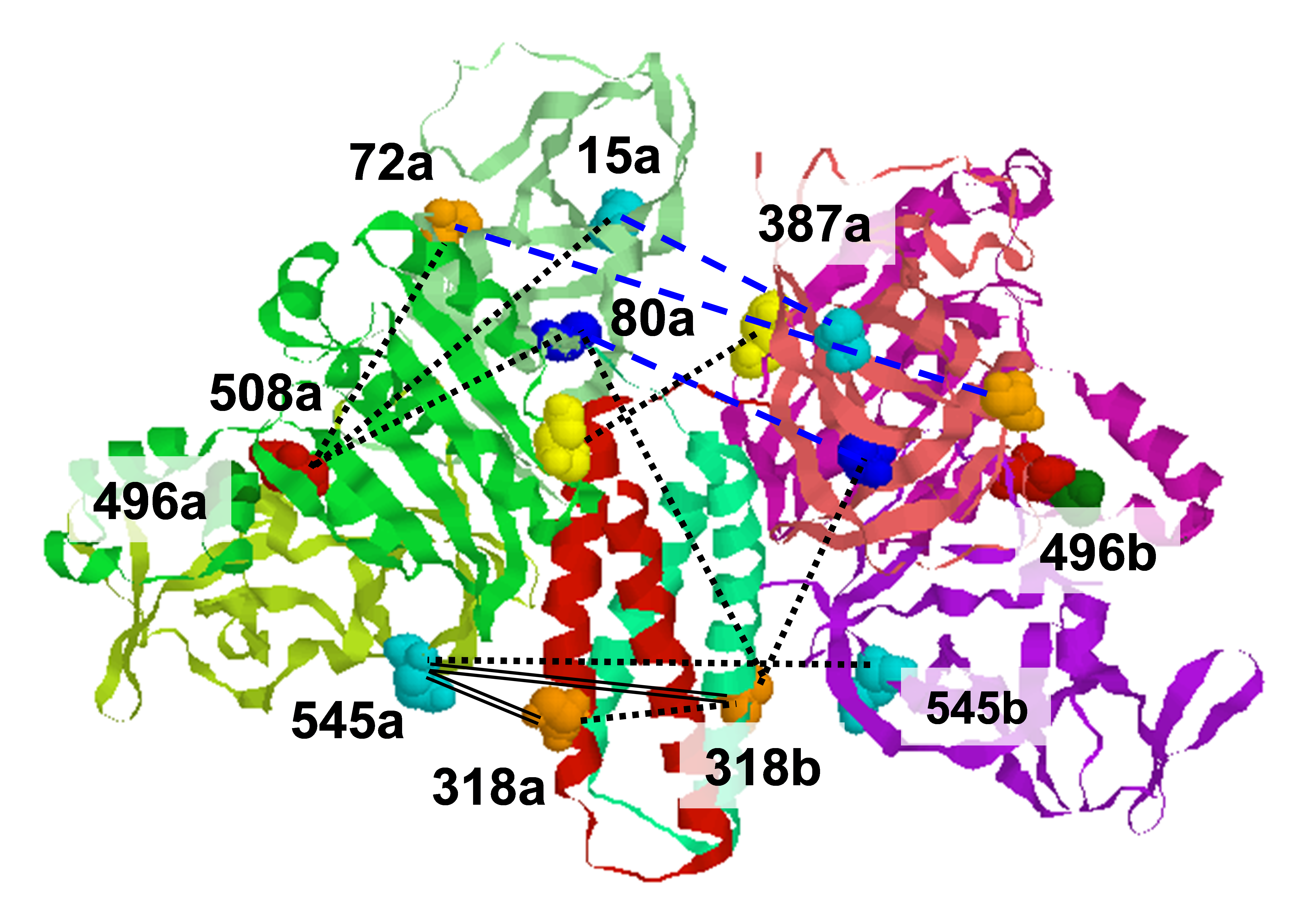
Rigid Body Refinement of Protein Complexes with Long-Range Distance Restraints from Pulsed Dipolar ESR
J. Bhatnagar, J.H. Freed, and B.R. Crane.
In Two-Component Signaling Systems, Part B, M. Simon, B.R. Crane and A.B. Crane, Eds.
Methods in Enzymology 423, Ch. 4, pp. 117-133 (2007).
<doi: 10.1016/S0076-6879(07)23004-6>
PMID:
17609128
|

|
|
ABSTRACT: The modeling of protein–protein complexes greatly benefits from the incorporation of experimental distance restraints. Pulsed dipolar electron spin resonance spectroscopy is one such powerful technique for obtaining long-distance restraints in protein complexes. Measurements of the dipolar interaction between two spins placed specifically within a protein complex give information about the spin–spin separation distance. We have developed a convenient method to incorporate such long-range distance information in the modeling of protein–protein complexes that is based on rigid body refinement of the protein components with the software Crystallography and NMR System (CNS). Factors affecting convergence such as number of restraints, error allocation scheme, and number and position of spin labeling sites were investigated with real and simulated data. The use of 4 to 5 different labeling sites on each protein component was found to provide sufficient coverage for producing accuracies limited by the uncertainty in the spin-label conformation within the complex. With an asymmetric scheme of allocating this uncertainty, addition of simulated restraints revealed the importance of longer distances within a limited set of total restraints. We present two case studies: (1) refinement of the complex formed between the histidine kinase CheA and its coupling protein CheW, and (2) refinement of intra-helical separations in the protein a-synuclein bound to micelles.
|
|
|
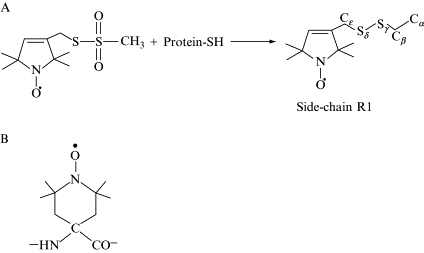
Measuring Distances by Pulsed Dipolar ESR Spectroscopy: Spin-Labeled Histidine Kinases
P.P. Borbat and J.H. Freed.
In Two-Component Signaling Systems, Part B, M. Simon, B.R. Crane and A.B. Crane, Eds.
Methods in Enzymology 423, Ch. 3, pp. 52-116 (2007).
<doi: 10.1016/S0076-6879(07)23003-4>
PMID:
17609127
|
|
|
ABSTRACT: Applications of dipolar ESR spectroscopy to structural biology are rapidly expanding, and it has become a useful method that is aimed at resolving protein structure and functional mechanisms. The method of pulsed dipolar ESR spectroscopy (PDS) is outlined in the first half of the chapter, and it illustrates the simplicity and potential of this developing technology with applications to various biological systems. A more detailed description is presented of the implementation of PDS to reconstruct the ternary structure of a large dimeric protein complex from Thermotoga maritima, formed by the histidine kinase CheA and the coupling protein CheW. This protein complex is a building block of an extensive array composed of coupled supramolecular structures assembled from CheA/CheW proteins and transmembrane signaling chemoreceptors, which make up a sensor that is key to controlling the motility in bacterial chemotaxis. The reconstruction of the CheA/CheW complex has employed several techniques, including X-ray crystallography and pulsed ESR. Emphasis is on the role of PDS, which is part of a larger effort to reconstruct the entire signaling complex, including chemoreceptor, by means of PDS structural mapping. In order to precisely establish the mode of coupling of CheA to CheW and to globally map the complex, approximately 70 distances have already been determined and processed into molecular coordinates by readily available methods of distance geometry constraints.
|
|
|
Pros and Cons of Pulse Dipolar ESR: DQC and DEER
P.P. Borbat and J.H. Freed.
EPR Newsletter 17, 21-33 (2007).
|

|
|
INTRODUCTION: Continuous-wave (cw) and pulsed ESR have been extensively applied to biological problems in the context of molecular dynamics and are now increasingly applied to study biomolecular structure and function. cw ESR has been used to measure distances in the range of 6–20 Å between pairs of nitroxide spin labels. Distance measurements using pulse ESR methods, a major advance in this area, are currently able to deliver long-distance constraints in the range of 10–80 Å. The distance constraints from pulse ESR can for example be used to establish protein folding or orient and dock proteins and their subunits, yielding useful insights into the structure of a protein or a protein complex. They can also aid in refinement of NMR data. We refer to this emerging methodology as 'pulsed dipolar (ESR) spectroscopy' or PDS for short.
|
|
|
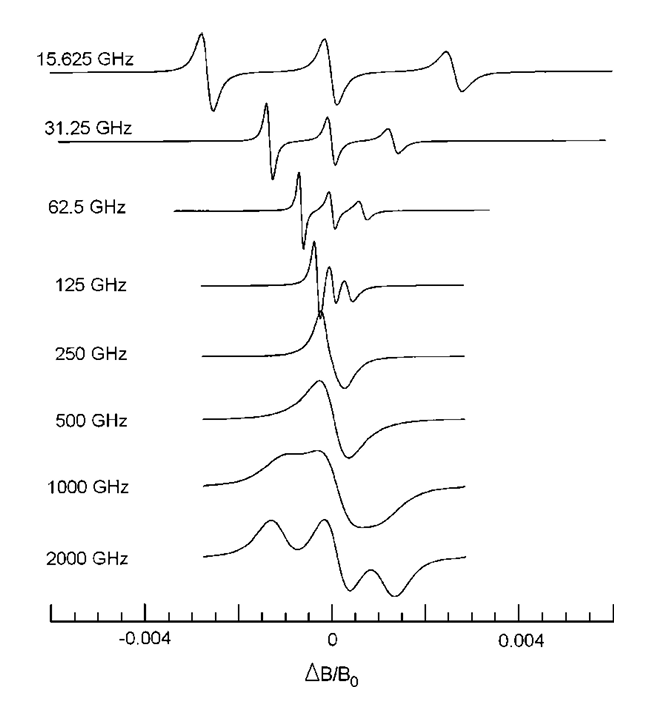
High-Frequency ESR at ACERT
K.A. Earle, B. Dzikovski, W. Hofbauer, J.K. Moscicki and J.H. Freed.
Magn. Reson. Chem. 43, S256-S266 (2005).
|
|
|
High-Frequency ESR at ACERT
K.A. Earle, B. Dzikovski, W. Hofbauer, J.K. Moscicki and J.H. Freed.
Magn. Reson. Chem. 43, S256-S266 (2005).
<doi: 10.1002/mrc.1684>
PMID:
16235203
|

|
|
ABSTRACT: High-field ESR offers many advantages in exploring fundamental questions of structure and dynamics in chemical, biological and physical samples. We provide a review of recent work performed at ACERT demonstrating the utility and flexibility of our methods for extracting both qualitative and quantitative information from a variety of systems. In particular, we emphasize the utility of multi-frequency ESR techniques for unraveling the details of the complex dynamical modes of proteins in solution and in heterogeneous systems such as lipid bilayers. We also include indications of directions for future work where appropriate.
|
|
|
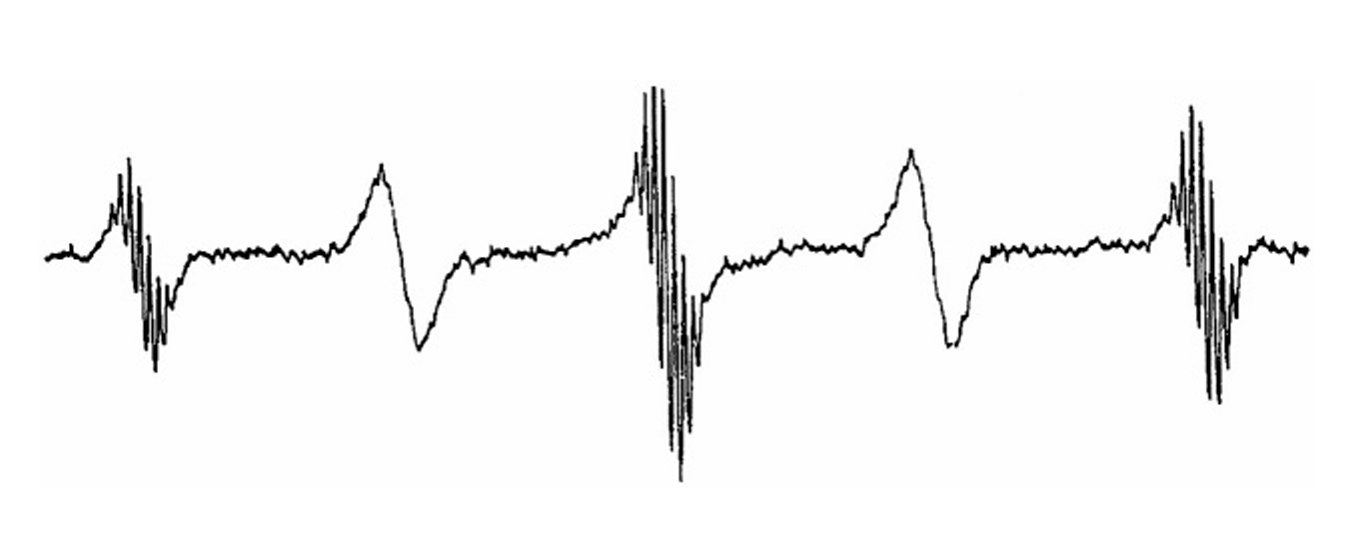
ESR and Molecular Dynamics
J.H. Freed.
In Biomedical EPR, Part B: Methodology, Instrumentation and Dynamics, S.S. Eaton, G.R. Eaton, and L.J. Berliner, Eds.
Biological Magnetic Resonance 24, Chapter 9, pp. 239-268 (2005). (Kluwer, NY).
|
|
|
ESR and Molecular Dynamics
J.H. Freed.
In Biomedical EPR, Part B: Methodology, Instrumentation and Dynamics, S.S. Eaton, G.R. Eaton, and L.J. Berliner, Eds.
Biological Magnetic Resonance 24, Chapter 9, pp. 239-268 (2005). (Kluwer, NY).
<doi: 10.1007/0-306-48533-8_9>
|

|
|
ABSTRACT: The development of ESR for the study of spin-relaxation and molecular dynamics of organic radicals and spin labels in fluids is reviewed from a historical perspective.
|
|
|
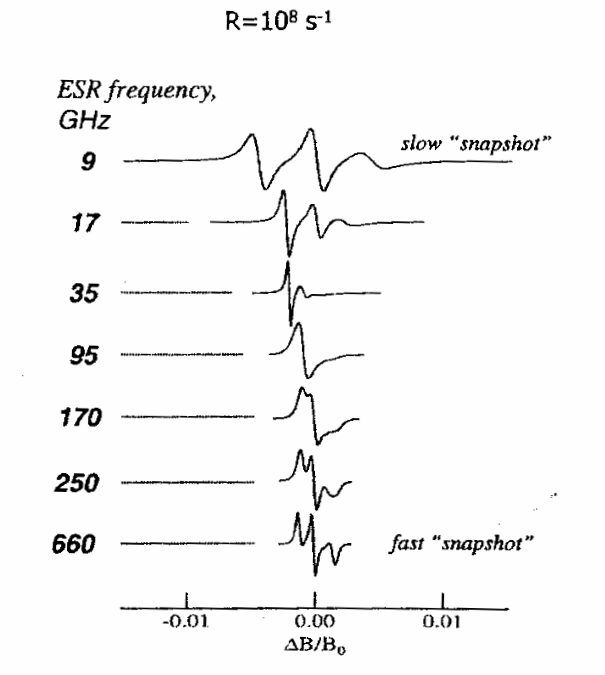
High Field ESR: Applications to Protein Structure and Dynamics: HF ESR Protein Structure and Dynamics
K.A. Earle and A.I. Smirnov.
In Very High Frequency (VHF) ESR/EPR, O. Grinberg and L.J. Berliner, Eds.
Biological Magnetic Resonance 22, Chapter 4, pp. 95-143 (2004). (Kluwer, NY).
<doi: 10.1007/978-1-4757-4379-1_4>
|
|
|
ABSTRACT: Electron Spin Resonance at high magnetic fields (HF ESR) is rapidly developing into a powerful biophysical tool which is uniquely positioned to address complex aspects of structure and dynamics of proteins, membranes, and macromolecular assemblies at molecular level. The source of the contemporary resurgence of interest in ESR as a biophysical tool is that there are, broadly speaking, three large groups of problems, which can be approached with ESR methods but cannot easily be studied by traditional structural techniques: (1) structure and dynamics of large molecular weight proteins in solution; (2) membrane and membrane-associated proteins: structure, location with respect to the membrane, side-chain dynamics, and interactions with other membrane components or DNA's and RNA's; (3) fast conformational transitions of proteins and RNA's in solution, protein folding and re-folding. The focus of this review is mainly on HF ESR spin-labeling techniques, primarily via nitroxide spin labels, as this method is the most flexible and is even applicable to proteins which are otherwise ESR-silent. We start with physical aspects of ESR of nitroxide spin labels at high magnetic fields in order to categorize the characteristic information that can be gained from such experiments. Then we describe practical applications of spin-labeling HF ESR to study structure and dynamics of complex biophysical systems. We also discuss several details of the ESR motional theory based on the stochastic Liouville equation (SLE) that are relevant to nitroxide line shape analysis in order to appreciate both the information available from and the limitations of the method. Particular emphasis is given to multifrequency HF ESR methods in studies of spin-labeled membranes and biopolymers. Finally, we review the use of HF ESR in applications to molecular structure including distance measurements, determination of molecular orientations from ESR of ordered samples, and structural studies based on g-factor measurements.
|
|
|
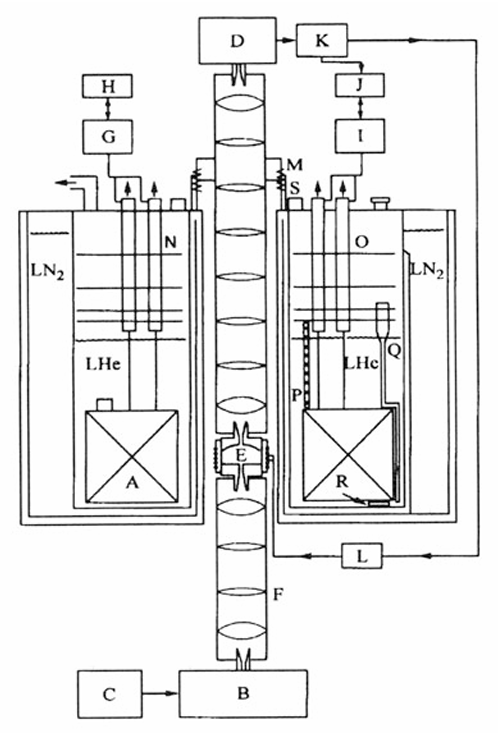
The Development of High Field/High Frequency ESR: Historical Overview
J.H. Freed.
In Very High Frequency (VHF) ESR/EPR, O. Grinberg and L.J. Berliner, Eds.
Biological Magnetic Resonance 22, Chapter 2, pp. 19-43 (2004). (Kluwer, NY).
<doi: 10.1007/978-1-4757-4379-1_2>
|
|
|
ABSTRACT: We discuss the development of high field ESR into a powerful and flexible tool for studies of structure and dynamics in a wide variety of systems including those of biological interest. A range of techniques are discussed with particular emphasis on the developments at Cornell University, but the contributions of other groups to the constant refinement of the state of the art are also noted.
|
|
|
|
|
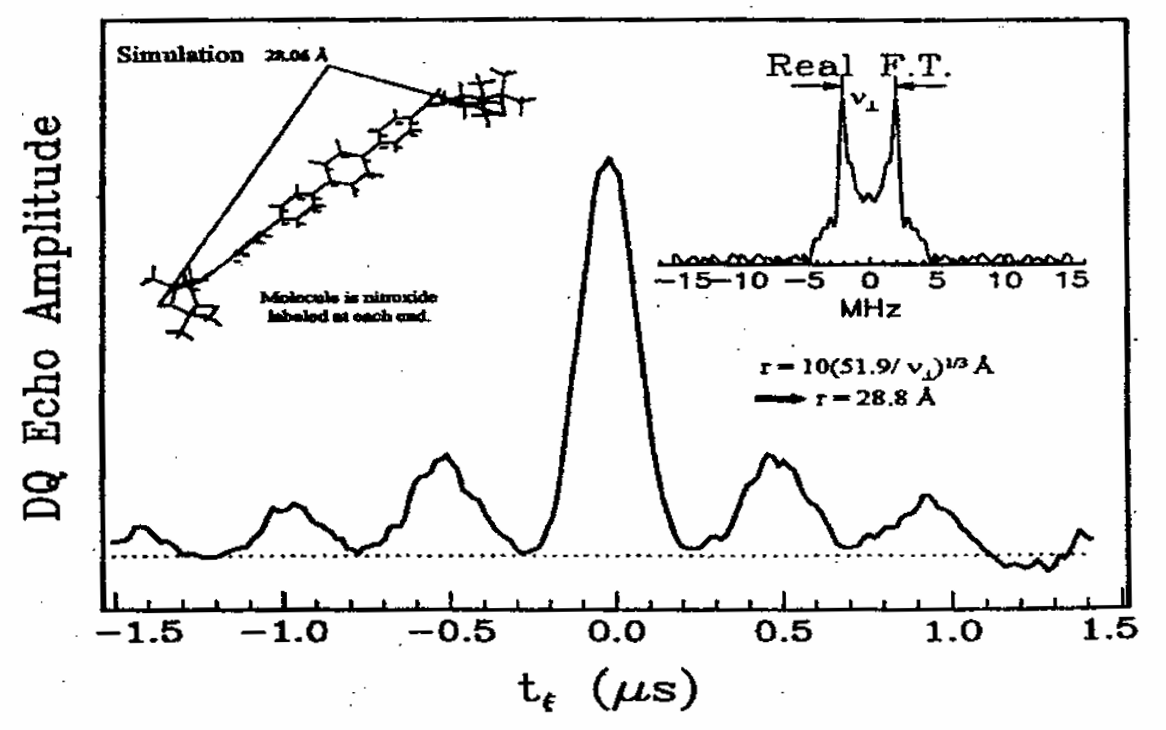
Modern ESR Methods in Studies of the Dynamic Structure of Proteins and Membranes
J.H. Freed.
In EPR in the 2lst. Century , A. Kawamori, J. Yamauchi, and H. Ohta, Eds.
Elsevier Science, Amsterdam, Netherlands, 719-730 (2002).
<doi: 10.1016/B978-044450973-4/50117-7>
|
|
|
ABSTRACT: A review of modern electron spin resonance (ESR) techniques for studying the dynamic structure of proteins and membranes using nitroxide spin labels is presented. In particular multiple quantum coherence FT-ESR, multifrequency ESR based on high frequency ESR and two dimensional ESR to study the structure and dynamics of bio-membranes and proteins are illustrated by several examples. Distances in biomolecules have been determined accurately. The details of complex dynamics in proteins and the dynamic structure of membranes were characterized.
|
|
|
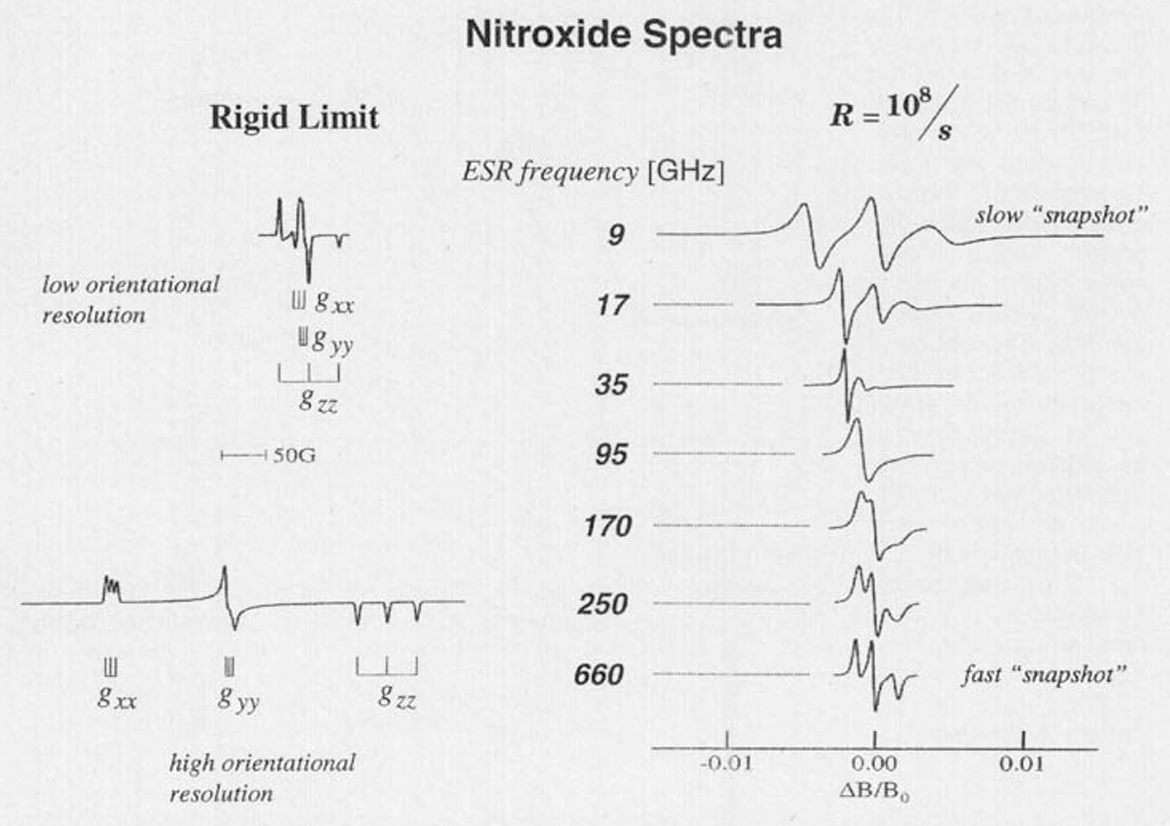
Electron Spin Resonance in Studies of Membranes and Proteins
P.P. Borbat, A.J. Costa-Filho, K.A. Earle, J.K. Moscicki, J.H. Freed.
Science 291, 266-269, (2001).
<doi: 10.1126/science.291.5502.266>
PMID:
11253218
|
|
|
ABSTRACT: We provide a review of current electron spin resonance (ESR) techniques for studying basic molecular mechanisms in membranes and proteins by using nitroxide spin labels. In particular, nitroxide spin label studies with high-field/high-frequency ESR and two-dimensional Fourier transform ESR enable one to accurately determine distances in biomolecules, unravel the details of the complex dynamics in proteins, characterize the dynamic structure of membrane domains, and discriminate between bulk lipids and boundary lipids that coat transmembrane peptides or proteins; these studies can also provide time resolution to studies of functional dynamics of proteins. We illustrate these capabilities with recent examples.
|
|
|
New Technologies in Electron Spin Resonance
J.H. Freed.
Annu. Rev. Phys. Chem. 51, 655-689 (2000).
<doi: 10.1146/annurev.physchem.51.1.655>
PMID:
11031296
|
|
|
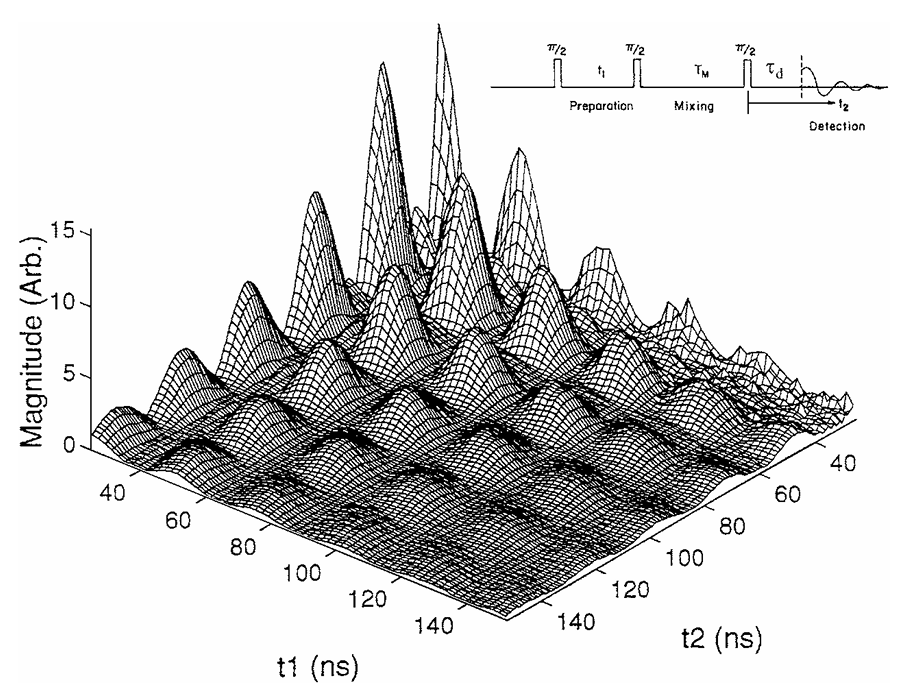
ABSTRACT: New electron spin resonance (ESR) technologies have been developed, which have led to new and improved applications. (a) The development of two-dimensional Fourier transform (FT) ESR required spectrometers providing intense π∕2 microwave pulses of very short (3–5 ns) duration, wide bandwidths, and very short dead times. It has enabled studies that resolve sophisticated details of molecular dynamics in complex fluids. (b) Methods that produce multiple quantum coherences by pulsed ESR now enable accurate measurements of large distances (>12Å). (c) One of the most important advances has been the extension of ESR to high magnetic fields and high frequencies. This has benefited from the utilization of quasi-optical methods, especially above 150 GHz. The greatly improved orientational resolution and the faster "snapshot" of motions that are provided by ESR at high frequencies enhance studies of molecular dynamics. The use of both high and lower frequencies enables one to unravel faster and slower modes from the complex dynamics of fluids and macromolecules. (d) The development of FT-ESR imaging required substantial pulsed field gradients lasting only 50–100 ns. ESR imaging is effective in studying diffusion in fluids. Areas for further development are also described.
|
|
|
Double Quantum ESR and Distance Measurements
P.P. Borbat and J.H. Freed.
In Distance Measurements in Biological Systems by EPR, L.J. Berliner, S.S. Eaton, and G.R, Eaton, Eds.
Biological Magnetic Resonance 19, Chapter 9, pp. 383-459 (2000). (Kluwer, NY).
|
|
|
Double Quantum ESR and Distance Measurements
P.P. Borbat and J.H. Freed.
In Distance Measurements in Biological Systems by EPR, L.J. Berliner, S.S. Eaton, and G.R, Eaton, Eds.
Biological Magnetic Resonance 19, Chapter 9, pp. 383-459 (2000). (Kluwer, NY).
<doi: 10.1007/0-306-47109-4_9>
|

|
|
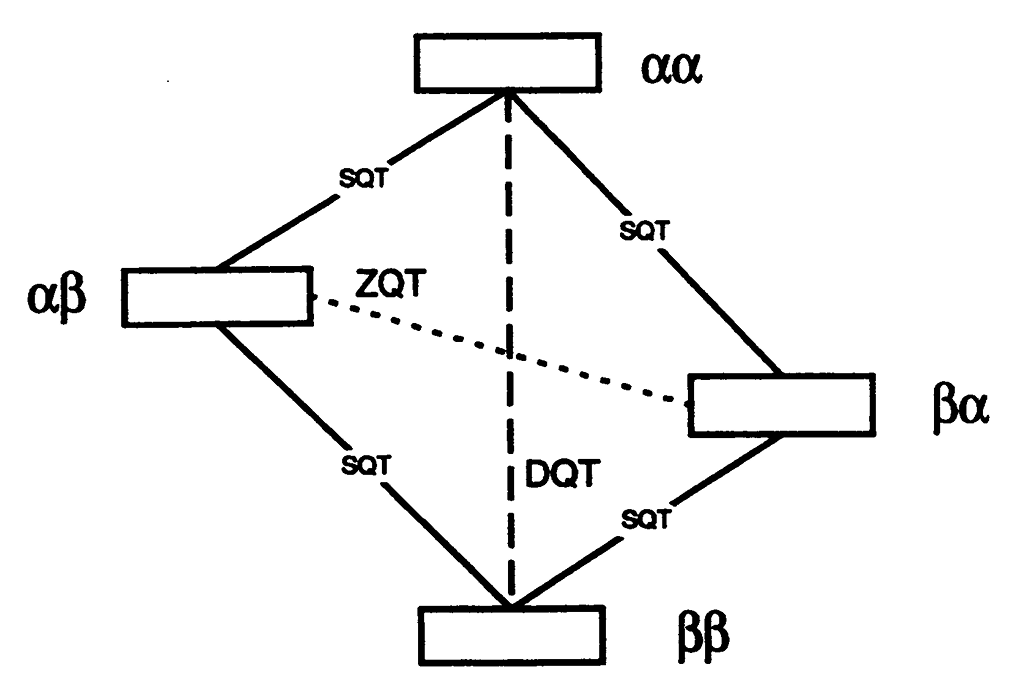
ABSTRACT: "Allowed" double quantum coherences (DQC) can now be routinely generated in disordered and oriented solids containing nitroxide biradicals and random distributions of stable radicals. The Pake doublets obtained from DQC pathways can be effectively used to determine a broad range of distances in the former case whereas decay constants yield concentrations in the latter. The DQC signals are strong and often comparable to standard single quantum signals. They are free of any large undesirable signals, so the DQ experiment is easy to perform. Their strong intensity permits the study of low concentrations of spins in samples typical of those ordinarily met in the case of doubly-labeled macromolecules such as proteins and polypeptides. The upper range of distances for systems labeled with nitroxides is estimated to be ca. 80 Å. In the limit of non-selective pulses the interpretation of DQC signals becomes independent of complicating geometric features which affect other ESR distance methods. The method is compared to other existing pulse distance measurement techniques and future improvements are also discussed.
|
|
© 2022
|
.svg)